Multiple Choice
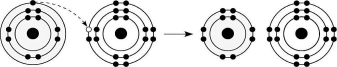 Figure 2.6
Figure 2.6
-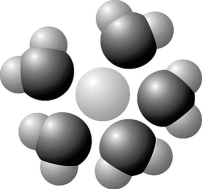 Figure 2.7
Figure 2.7
Based on your knowledge of the polarity of water molecules, the solute molecule depicted in Figure 2.7 is most likely
A) positively charged.
B) negatively charged.
C) without charge.
D) hydrophobic.
E) nonpolar.
Correct Answer:

Verified
Correct Answer:
Verified
Q42: Which of the following takes place as
Q42: Many mammals control their body temperature by
Q52: An atom with atomic number 12 would
Q75: Which of the following correctly describes chemical
Q76: The molar mass of glucose (C₆H₁₂O₆) is
Q78: Nitrogen (N) is much more electronegative than
Q79: Glucose has a molecular mass of 180
Q83: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6148/.jpg" alt=" Figure 2.6 -What
Q84: Molybdenum has an atomic number of 42.
Q85: How many glucose molecules are contained in