Multiple Choice
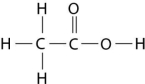 Figure 2.8
Figure 2.8
-How many grams of the compound in Figure 2.8 would be required to make 1 L of a 0.5 M solution?
(carbon = 12, oxygen = 16, hydrogen = 1)
A) 29
B) 30
C) 60
D) 150
E) 342
Correct Answer:

Verified
Correct Answer:
Verified
Q40: An equal volume (5 mL)of vinegar from
Q61: Trace elements are those required by an
Q86: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7910/.jpg" alt=" Figure 2.4 -In
Q126: What is the hydroxyl ion (OH-) concentration
Q127: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6148/.jpg" alt=" Figure 2.3 -Which
Q128: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6148/.jpg" alt=" Figure 2.4 -How
Q130: How many electron pairs are shared between
Q133: In an aqueous solution, water molecules associate
Q135: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6148/.jpg" alt=" Figure 2.3 -Which
Q136: Two atoms that have the same mass