Multiple Choice
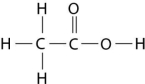 Figure 2.8
Figure 2.8
-How many grams of the compound in Figure 2.8 would be required to make 2.5 L of a 1 M solution?
(carbon = 12, oxygen = 16, hydrogen = 1)
A) 29
B) 30
C) 60
D) 150
E) 342
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q23: In ammonium chloride salt (NH4Cl), the anion
Q25: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6148/.jpg" alt=" Figure 2.3 -Which
Q27: If the pH of a solution is
Q29: What is the hydrogen ion (H+) concentration
Q30: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6148/.jpg" alt=" Figure 2.6 -Which
Q31: Oxygen has an atomic number of 8
Q35: We can be sure that a mole
Q36: Which of the following statements correctly describes
Q63: Fluorine has an atomic number of 9
Q74: Which of the following correctly describes any