Multiple Choice
Carbon dioxide (CO₂) is readily soluble in water, according to the equation CO₂ + H₂O ↔ H2CO3. Carbonic acid (H2CO3) is a weak acid. If CO₂ is bubbled into a beaker containing pure, freshly distilled water, which of the following graphs correctly describes the results?
A) 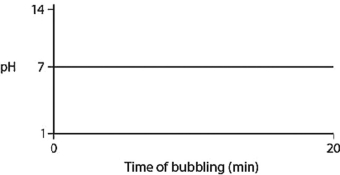
B) 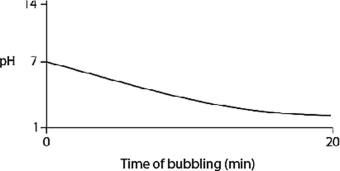
C) 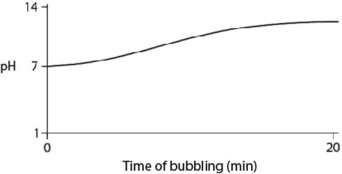
D) 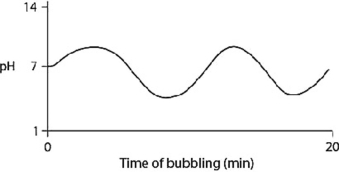
E) 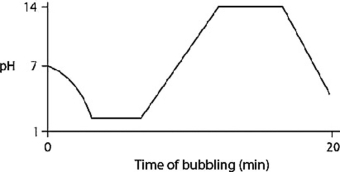
Correct Answer:

Verified
Correct Answer:
Verified
Q1: Which type of bond must be broken
Q15: An atom has 6 electrons in its
Q46: A 0.01 M solution of a substance
Q47: An ionic bond is formed by<br>A)sharing of
Q49: Which of the following effects is produced
Q58: A strong acid like HCl<br>A)dissociates completely in
Q91: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7910/.jpg" alt=" Figure 2.4 -In
Q95: Argon has atomic number 18. Which of
Q98: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6148/.jpg" alt=" Figure 2.6 -Which
Q100: Buffers are substances that help resist shifts