Multiple Choice
Which statement concerning the crystal field theory of octahedral transition metal complexes is not correct?
A) The crystal field splitting (o) increases with increasing charge of the metal ion for the same set of ligands.
B) The crystal field splitting (o) is determined solely by the charge of the transition metal complex ion.
C) The 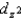 and
and 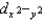 orbitals are higher in energy than the dxy, dxz, and dyz orbitals because these two point directly toward the ligands.
orbitals are higher in energy than the dxy, dxz, and dyz orbitals because these two point directly toward the ligands.
D) All ligands contribute to the strength of the crystal field.
E) The ordering of the orbitals, 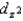 and
and 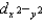 are higher in energy than the dxy, dxz, and dyz orbitals, is independent of the strength of the crystal field.
are higher in energy than the dxy, dxz, and dyz orbitals, is independent of the strength of the crystal field.
Correct Answer:

Verified
Correct Answer:
Verified
Q88: Which of the following square planar complexes
Q89: The effects of lead poisoning can sometimes
Q90: Which of the following is a chelation
Q91: What is the correct formula for sodium
Q92: Which of the following complexes has the
Q94: Which pieces of information are needed to
Q95: Cisplatin and other metallodrugs used with cancer
Q96: Porphyrins are polydentate ligands found in nature.
Q97: How many unpaired electrons are there in
Q98: A solution containing an octahedral metal ion