Multiple Choice
Which d orbital(s) is/are lowest in energy in a tetrahedral complex?
A) dz2
B) dxy, dxz, and dyz
C) dz2 and 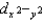
D) 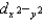
E) dxy and dxz
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q45: The d<sub>xy</sub>, d<sub>xz</sub>, and d<sub>yz</sub> orbitals are
Q46: Anemia is associated with a deficiency of
Q47: How many unpaired spins are there in
Q48: Which 3d orbitals have a higher energy
Q49: Why are compounds of most of the
Q51: What is the shape of the complex
Q52: How many unpaired electrons are there in
Q53: The correct name for [Fe(NH<sub>3</sub>)<sub>4</sub>(H<sub>2</sub>O)<sub>2</sub>][NiCl<sub>4</sub>] is _<br>A)tetraamminodiaquoiron(II)
Q54: The coordination number for nickel in the
Q55: The coordination number around the Ni ion