Multiple Choice
In a tetrahedral crystal field, ________
A) dxy, dxz, and dyz are lower in energy than 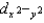 and dz2.
and dz2.
B) 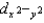 and dz2 are lower in energy than dxy, dxz, and dyz.
and dz2 are lower in energy than dxy, dxz, and dyz.
C) dxy, dxz, dyz, 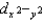 , and dz2 all have different energies.
, and dz2 all have different energies.
D) dxy, dxz, dyz, 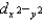 , and dz2 all have the same energy.
, and dz2 all have the same energy.
E) dxy, dxz, and dyz have the same energy, and 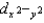 and dz2 have different energies.
and dz2 have different energies.
Correct Answer:

Verified
Correct Answer:
Verified
Q5: Explain why Ni(CN)<sub>4</sub><sup>2</sup><font face="symbol"><sup></sup></font> is diamagnetic and
Q6: Define the term polydentate ligand, and give
Q7: Which statement describing the splitting of metal
Q8: What is the correct formula for pentaammineaquairon(II)
Q9: Which atoms on EDTA bond to metal
Q11: Transition metal ions often absorb visible light
Q12: As the value of the crystal field
Q13: When the five 3d orbitals on a
Q14: Which statement A-D regarding isomers in transition
Q15: A strong crystal field produces a large