Multiple Choice
Which statement A-D concerning the crystal field theory of tetrahedral transition metal complexes is not correct?
A) The crystal field splitting (o) increases with increasing charge of the metal ion for the same set of ligands.
B) The dxy, dxz, and dyz orbitals are higher in energy than the dz2 and 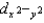 orbitals.
orbitals.
C) The crystal field splitting (o) depends on the nature of the ligands and their positions in the spectrochemical series.
D) The lobes of the dxy, dxz, and dyz orbitals are closer to the ligands at the four corners of the tetrahedron than are the lobes of the dz2 and dx2 y2 orbitals.
E) Statements A-D all are correct.
Correct Answer:

Verified
Correct Answer:
Verified
Q102: How many stereoisomers are there for the
Q103: For the coordination compound [Co(NH<sub>3</sub>)<sub>4</sub>Cl<sub>2</sub>]NO<sub>3</sub>, identify<br>(1) the
Q104: Consider two complexes: (I) [Fe(CN)<sub>6</sub>]<sup>4</sup><font face="symbol"><sup></sup></font>, and
Q105: Which statement concerning ligands in transition metal
Q106: CoF<sub>6</sub><sup>3</sup><font face="symbol"><sup></sup></font> absorbs light in the red
Q108: Which d orbital(s) is/are highest in energy
Q109: The correct formula for ammonium tetracyanoplatinate(II) is
Q110: The correct name for the compound [Fe(NH<sub>3</sub>)<sub>5</sub>H<sub>2</sub>O](NO<sub>3</sub>)<sub>2</sub>
Q111: The coordination number for copper in the
Q112: Chlorophyll a is a pigment in plants