Essay
Ingestion of mercury, which is poisonous, often is treated using chelation therapy. One agent used in this therapy is dimercaprol, which is shown below. Identify the features in the structure of this molecule that make it an effective chelation agent. 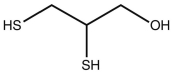
Correct Answer:

Verified
The sulfur and oxygen atoms on this liga...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q36: Iron accumulation in the human body has
Q37: Complex ions with different ligands have different
Q38: In K<sub>4</sub>[Fe(CN)<sub>6</sub>], the counter ion is _,
Q39: The correct name for [Co(NH<sub>3</sub>)<sub>5</sub>Cl]Cl<sub>2</sub> is _<br>A)pentaamminechlorocobalt(III)
Q40: Given that the observed absorption maximum for
Q42: Consider two complex ions: (I) [Co(CN)<sub>6</sub>]<sup>3</sup><font face="symbol"><sup></sup></font>,
Q43: How many d electrons are there in
Q44: Co(NH<sub>3</sub>)<sub>6</sub><sup>3</sup><font face="symbol"><sup></sup></font>(aq) absorbs light in the blue
Q45: The d<sub>xy</sub>, d<sub>xz</sub>, and d<sub>yz</sub> orbitals are
Q46: Anemia is associated with a deficiency of