Multiple Choice
Different concentrations of ions on either side of a cell membrane constitute a concentration cell, which is described by the Nernst equation given below. What is the electrochemical potential across a membrane when the sodium ion concentration is 0.10 M on one side of the membrane and 0.010 M on the other side, and the temperature is 37C (body temperature) ? (Given: 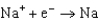 ,
, 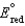 2.71 V; F 96,485 C; 1 J 1 C V; R 8.315 J / (mol K) )
2.71 V; F 96,485 C; 1 J 1 C V; R 8.315 J / (mol K) )
E E - 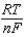 InQ
InQ
A) 61.5 mV
B) 26.7 mV
C) 2.65 V
D) 2.68 V
E) 2.98 V
Correct Answer:

Verified
Correct Answer:
Verified
Q53: Draw two possible Lewis structures of the
Q54: Hearing aid batteries utilize a zinc/air electrochemical
Q55: The isotope <sup>18</sup>F is used in a
Q56: The ion found in chlorophyll is _<br>A)Zn<sup>2</sup><font
Q57: Why are the radioactive isotopes of cesium
Q59: The concentration of a major essential element
Q61: Enzymes in plants convert nitrate into ammonia.
Q62: Superoxide dismutase is an enzyme that converts
Q63: The isotope <sup>201</sup>Th is used in the
Q88: A patient is injected with a 5.0