Multiple Choice
Hydroxyapatite, which is a component of tooth enamel, is very insoluble in water. For the following reaction, Ksp 2.3 1059. 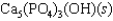
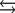
 What is the molar concentration of Ca5(PO4) 3 when the pH of the mouth is 4.0?
What is the molar concentration of Ca5(PO4) 3 when the pH of the mouth is 4.0?
A) 2.3 1049 M
B) 2.3 1059 M
C) 2.3 1055 M
D) 2.3 1063 M
E) 0, teeth don't dissolve!
Correct Answer:

Verified
Correct Answer:
Verified
Q59: The concentration of a major essential element
Q61: Enzymes in plants convert nitrate into ammonia.
Q62: Superoxide dismutase is an enzyme that converts
Q63: The isotope <sup>201</sup>Th is used in the
Q65: Plants convert nitrogen into ammonia. This biological
Q66: A radioactive isotope that emits the shortest
Q67: Which metal, by the number of atoms,
Q68: Thallium-201 is used in cardiac imaging. It
Q69: Lithium is used in the treatment of
Q88: A patient is injected with a 5.0