Multiple Choice
The diagram below represents a voltaic cell. What is the balanced electrochemical reaction represented by this cell? Al(s) |Al3(1.0 M) ||Cu2(1.0 M) |Cu(s)
A) 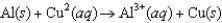
B) 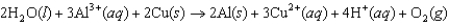
C) 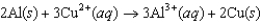
D) 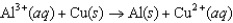
E) 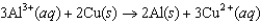
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q149: Copper is oxidized by nitric acid. If
Q150: The standard hydrogen electrode is _<br>A)used to
Q151: In a classroom demonstration of a redox
Q152: The Energizer Bunny in the television commercial
Q153: Which statement is not correct for a
Q155: Proteins containing a certain functional group (identified
Q156: What is the cell potential for a
Q157: The capacity of a battery usually is
Q158: The diagram below represents a voltaic cell.
Q159: Chromium often is electroplated on other metals