Multiple Choice
Which statement about a voltaic cell is not correct?
A) The standard reduction potential is a measure of the propensity for a reduction half-reaction to occur when concentrations are 1 M, the pressure is 1 atm, and the temperature is 298 K.
B) A reduction half-reaction with a standard reduction potential, 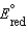 , when reversed becomes an oxidation half-reaction with
, when reversed becomes an oxidation half-reaction with 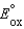
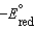 .
.
C) The standard cell potential, 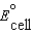 , is a measure of the electromotive force (emf) generated by the cell reactions at 298 K when concentrations are 1 M and the pressure is 1 atm.
, is a measure of the electromotive force (emf) generated by the cell reactions at 298 K when concentrations are 1 M and the pressure is 1 atm.
D) The emf of a cell is a measure of the force with which the cell pumps electrons from the cathode to the anode.
E) The cell voltage measured with a voltmeter is not necessarily the standard cell potential.
Correct Answer:

Verified
Correct Answer:
Verified
Q143: Which statement about corrosion is not correct?<br>A)Corrosion
Q144: What kind of chemical reaction occurs at
Q145: Glancing at a periodic table, where do
Q146: Silver tarnishes due to the formation of
Q147: When a voltaic cell reaches equilibrium, _<br>A)
Q149: Copper is oxidized by nitric acid. If
Q150: The standard hydrogen electrode is _<br>A)used to
Q151: In a classroom demonstration of a redox
Q152: The Energizer Bunny in the television commercial
Q153: Which statement is not correct for a