Multiple Choice
The oxidation of methanol, as described by the equation below, has a G value of 937.9 kJ/mol. What is the standard cell potential for a methanol fuel cell? 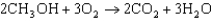
A) 0.405 V
B) 9.72 V
C) 0.810 V
D) (2.43 V)
E) (9.72 V)
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q103: For the following electrochemical cell, draw and
Q104: Based on the information in the table
Q105: The unit of current, ampere (A), is
Q106: Where in the periodic table do you
Q107: A hydrogen fuel cell depends on _<br>A)fusion
Q109: The Nernst equation can be used to
Q110: For the following electrochemical cell, write the
Q111: Permanganate ion can oxidize sulfite in basic
Q112: Cadmium is a toxic heavy metal. The
Q113: Which one of the following statements about