Multiple Choice
The reaction 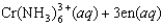
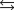
 where en represents ethylenediamine, has a small value for the enthalpy change, Hrxn, yet the free-energy change is large because ________
where en represents ethylenediamine, has a small value for the enthalpy change, Hrxn, yet the free-energy change is large because ________
A) the reaction rate is fast.
B) the entropy change is large and positive.
C) the enthalpy change is large enough to matter.
D) the entropy change is large and negative.
E) ethylenediamine has amino groups that are stronger bases than ammonia.
Correct Answer:

Verified
Correct Answer:
Verified
Q55: Hydrogen iodide can theoretically be made by
Q56: The standard molar enthalpy of fusion for
Q57: Name and state the first three laws
Q58: As T approaches infinity, ln K approaches
Q59: Which of the following states of motion
Q61: Which of the following is the best
Q62: According to the second law of thermodynamics,
Q63: Perfect crystals of carbon monoxide (CO) are
Q64: Which, if any, of statements A-D is
Q65: If for a given chemical reaction at