Multiple Choice
Determine 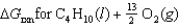
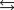
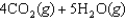 given the following information:
given the following information: 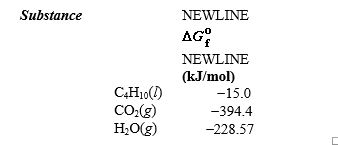
A) (2,705 kJ)
B) (608.0 kJ)
C) (1,791 kJ)
D) (3,457 kJ)
E) (608.0 kJ)
Correct Answer:

Verified
Correct Answer:
Verified
Q124: Before class, students were distributed throughout a
Q125: Indicate which of the following has the
Q126: A chemist is planning to study a
Q127: The standard entropy of N<sub>2</sub>(g) is 191.5
Q128: Determine <font face="symbol"></font><font face="symbol"></font> <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3835/.jpg" alt="Determine
Q130: Jane can accept that <font face="symbol"></font>G<font face="symbol"></font>
Q131: Using the thermodynamic data below, determine the
Q132: Noxious NO gas can form from N<sub>2</sub>
Q133: What is the standard entropy change when
Q134: When you increase the volume of a