Short Answer
Using the thermodynamic data below, determine the equilibrium constant for the conversion of oxygen to ozone at 2,000 K.
Substance 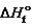 (kJ/mol)
(kJ/mol) 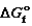 (kJ/mol) S (J/mol · K)
(kJ/mol) S (J/mol · K)
O2(g) 0 0 205.0
O3(g) 142.3 163.4 237.6
3O2(g) 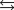 2O3(g)
2O3(g)
Correct Answer:

Verified
2.91 F1F1F...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q49: During a spontaneous chemical reaction, it is
Q50: The standard molar entropy of magnesium fluoride
Q51: Determine the value of <font face="symbol"></font>G<font face="symbol"></font>
Q52: The enthalpy of vaporization for toluene (C<sub>7</sub>H<sub>8</sub>)
Q53: Determine the standard entropy of N<sub>2</sub>(g) given
Q55: Hydrogen iodide can theoretically be made by
Q56: The standard molar enthalpy of fusion for
Q57: Name and state the first three laws
Q58: As T approaches infinity, ln K approaches
Q59: Which of the following states of motion