Multiple Choice
What is indicated by the shape of the titration curve? 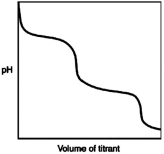
A) A diprotic acid was titrated with a strong base.
B) A triprotic acid was titrated with a strong base.
C) A diprotic base was titrated with a strong acid.
D) A triprotic base was titrated with a strong acid.
E) A strong acid was titrated with a strong base.
Correct Answer:

Verified
Correct Answer:
Verified
Q54: Which of the following species is least
Q55: Vitamin C is a monoprotic weak acid,
Q56: Which of the following cannot be mixed
Q57: The solubility product for an insoluble salt
Q58: Which of the following compounds contains a
Q60: One of the following solutions is acidic.
Q61: Bromocresol green is yellow in its acidic
Q62: The pK<sub>a</sub> of a weak acid was
Q63: A 25.0 mL solution of quinine was
Q64: The most suitable acid-base indicator for a