Multiple Choice
Calculate the equilibrium concentration of Zn2(aq) in a solution that is 0.0125 M Zn(NO3) 2 and 0.600 M NH3.
Given: Zn2(aq) 4NH3(aq) 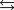 Zn(NH3) 42(aq) Kf 2.9 109.
Zn(NH3) 42(aq) Kf 2.9 109.
A) 7.8 1012 M
B) 4.7 1011 M
C) 1.5 108 M
D) 2.5 109 M
E) 2.9 109 M
Correct Answer:

Verified
Correct Answer:
Verified
Q115: Determine the molar concentration of Fe<sup>3</sup><font face="symbol"><sup></sup></font>(aq)
Q116: A solution of benzoic acid (0.21 M,
Q117: Acid-base indicators change color _<br>A)exactly when pH
Q118: A solution of nitrous acid (0.13 M,
Q119: In the following reaction, which species is
Q121: Let s be the number of moles
Q122: Dissolved concentrations of copper(II) as low as
Q123: A mining operation needs to separate silver
Q124: Glycolic acid, which is a monoprotic acid
Q125: A phosphate buffer solution (25.00 mL sample)