Multiple Choice
What is the equilibrium concentration of Cu(aq) in a solution that is 0.0200 M solution in CuNO3 and 0.450 M HCl(aq) ? Given: Cu(aq) 3Cl(aq) 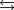 CuCl32 Kf 5 105.
CuCl32 Kf 5 105.
A) 1.0 107 M
B) 6.7 107 M
C) 4.4 107 M
D) 5.0 107 M
E) 5.0 105 M
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q45: Define the term Lewis base and provide
Q46: A solution of formic acid (0.15 M,
Q47: Hydrated transition metal ions produce solutions that
Q48: The pK<sub>a</sub> of a weak acid was
Q49: Which of the following represents the correct
Q51: What is the solubility of barium sulfate
Q52: Which statement A-D regarding acids and bases
Q53: The Yucca Mountain repository in Nevada, which
Q54: Which of the following species is least
Q55: Vitamin C is a monoprotic weak acid,