Multiple Choice
The acid ionization equilibrium constant, Ka, describes the reaction (where HA is a generic weak acid) ________
A) HA OH 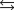 H2O A.
H2O A.
B) HA H2O 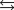 H3O A.
H3O A.
C) HA H3O 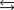 H2A H2O.
H2A H2O.
D) HA H2A 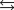 H2A HA.
H2A HA.
E) H3O A 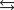 HA H2O.
HA H2O.
Correct Answer:

Verified
Correct Answer:
Verified
Q88: Aqueous solutions of _ are basic.<br>A)NaF<br>B)NaCl<br>C)NaBr<br>D)NH<sub>4</sub>I<br>E)KI
Q89: Which of the following is a strong
Q90: Solutions of each of the hypothetical bases
Q91: Which one of the following is a
Q92: What is the pH of a 0.35
Q94: What is the K<sub>a</sub> for nitrous acid,
Q95: A solution of the weak acid HF
Q96: Identify the weakest and strongest acids in
Q97: Diethylamine ((CH<sub>3</sub>CH<sub>2</sub>)<sub> </sub>NH<sub>2</sub>) is a weakly basic
Q98: Lactic acid is a major component of