Multiple Choice
Phosphoric acid is a triprotic acid, ionizing in the following sequential steps: H3PO4 H2O  H2PO4 H3O
H2PO4 H3O 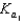 H2PO4 H2O
H2PO4 H2O  HPO42 H3O
HPO42 H3O 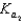 HPO42 H2O
HPO42 H2O  PO43 H3O
PO43 H3O 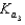 What is the Kb expression for the base, dihydrogen phosphate?
What is the Kb expression for the base, dihydrogen phosphate?
A) 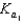
B) 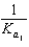
C) 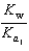
D) 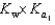
E) 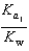
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q101: What is the pH of a 0.055
Q102: A substance that can act as both
Q103: Identify the weakest and strongest acids in
Q104: Methylamine (CH<sub>3</sub>NH<sub>2</sub>) is a weakly basic compound.
Q105: What is the pH of a 0.500
Q107: What is the concentration of the acetate
Q108: What is the concentration of [OH<font face="symbol"><sup></sup></font>]
Q109: What is the hydronium ion concentration of
Q110: A solution with a pH of 9.50
Q111: Which ending to the statement is not