Multiple Choice
Phosphoric acid is a triprotic acid, ionizing in the following sequential steps: H3PO4 H2O  H2PO4 H3O
H2PO4 H3O 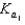 H2PO4 H2O
H2PO4 H2O  HPO42 H3O
HPO42 H3O 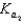 HPO42 H2O
HPO42 H2O  PO43 H3O
PO43 H3O  What is the Kb expression for the base, sodium phosphate?
What is the Kb expression for the base, sodium phosphate?
A) 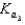
B) 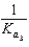
C) 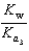
D) 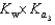
E) 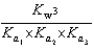
Correct Answer:

Verified
Correct Answer:
Verified
Q66: Ammonia (NH<sub>3</sub>) acts as a weak base
Q67: Sometimes liquid ammonia, NH<sub>3</sub>, is used as
Q68: Three acids found in foods are lactic
Q69: Which one of the following is not
Q70: Lactic acid is a major component of
Q72: Boric acid frequently is used as an
Q73: In the following reaction in aqueous solution,
Q74: Identify the weakest and strongest acids in
Q75: Which statement A-D is not correct? Pure
Q76: When [H<font face="symbol"><sup></sup></font>] <font face="symbol"></font> 4.0 <font