Multiple Choice
Phosphoric acid is a triprotic acid, ionizing in the following sequential steps: H3PO4 H2O  H2PO4 H3O
H2PO4 H3O 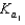 H2PO4 H2O
H2PO4 H2O  HPO42 H3O
HPO42 H3O 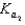
HPO42 H2O  PO43 H3O
PO43 H3O 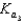 Which equilibrium is most important in determining the pH of a solution of sodium phosphate?
Which equilibrium is most important in determining the pH of a solution of sodium phosphate?
A) HPO42 H2O  PO43 H3O
PO43 H3O
B) H3PO4 H2O  H2PO4 H3O
H2PO4 H3O
C) PO43 H2O  HPO42 OH
HPO42 OH
D) H2PO4 H2O  H3PO4 OH
H3PO4 OH
E) 2H2O  H3O OH
H3O OH
Correct Answer:

Verified
Correct Answer:
Verified
Q37: In evaluating the pH of an aqueous
Q38: You decide to put your growing knowledge
Q39: The first disinfectant used by Joseph Lister
Q40: The analysis label on a 500 mL
Q41: Which one of the following salts forms
Q43: Which of the following groups, A-D, consist
Q44: Which one of the following is not
Q45: The concentration of acetic acid (pK<sub>a</sub> <font
Q46: A cleaning solution has a pOH of
Q47: Which expression defines the autoionization constant for