Multiple Choice
In the cesium chloride unit cell shown below, the cesium ions sit on the corners of a cube. What is the name of this unit cell? 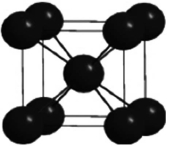
A) simple cubic
B) chloride-centered cubic
C) face-centered cubic
D) cubic-centered
E) body-centered cubic
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q120: If half of the tetrahedral holes in
Q121: Which of the following is true regarding
Q122: The cubic closest-packed crystal structure has a
Q123: Quartz, a form of SiO<sub>2</sub>, which contains
Q124: What type of crystal structure can produce
Q125: Platinum (Pt) has a face-centered cubic structure
Q127: Polonium crystallizes in a simple cubic pattern.
Q128: The center of a cubic hole is
Q129: Which is not true about a crystallographic
Q130: Draw a few subunits of the addition