Multiple Choice
In the diagram below, one beaker contains pure water and the other contains an equal volume of seawater. Seawater has various salts dissolved in it. The beakers are sitting in a totally enclosed chamber, and the outside temperature and pressure are held constant. Identify the statements below about this situation that are not correct. 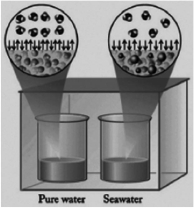
I. Water will be transferred from the pure water beaker to the seawater beaker.
II. Water will be transferred from the seawater beaker to the pure water beaker.
III.The vapor pressure of the pure water is higher than the vapor pressure of the seawater.
IV. Pure water evaporates at a faster rate than seawater.
V. Water in the gas phase condenses into both beakers at the same rate.
A) II only
B) II and V
C) II, IV, and V
D) II, III, and IV
E) I, III, and IV
Correct Answer:

Verified
Correct Answer:
Verified
Q26: Which of the solutions shown here will
Q27: Which solution, if either, would create the
Q28: Eugenol is a nonelectrolyte that contributes to
Q29: A solution is made by dissolving 100
Q30: Indicate which aqueous solution has the fastest
Q32: A solution is prepared by mixing 50
Q33: Which intermolecular interactions are likely to result
Q34: Predict which of the following ionic compounds
Q35: Rank the following ionic compounds in order
Q36: Why would you expect or predict sodium