Essay
Which of the following solutions, assuming equal volumes and a nonvolatile solute, would have the highest boiling point? Provide rationale for your choice. 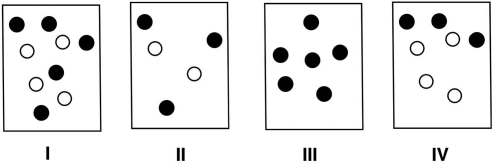
Correct Answer:

Verified
Solution I. Boiling point elevation is d...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
Solution I. Boiling point elevation is d...
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Related Questions
Q94: In the process of dialysis, a special
Q95: What mass of a 1.23 m sodium
Q96: The freezing point of a 0.060 m
Q97: A petroleum company separates benzene (78 g/mol)
Q98: Ion interaction energies are determined by Coulomb's
Q100: Indicate which aqueous solution has the lowest
Q101: Which of the following solutions, assuming equal
Q102: Which of the following ionic compounds would
Q103: Indicate which aqueous solution has the lowest
Q104: A physiological saline solution is 0.92% NaCl