Multiple Choice
At the point marked with a dot on the phase diagram, the solid will ________ 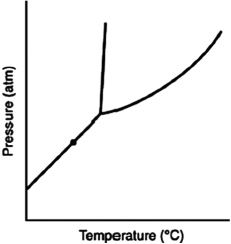
A) be indistinguishable from the gas.
B) boil.
C) melt.
D) sublime.
E) liquefy.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q68: Which of the following gases do you
Q69: For the following unsaturated phospholipid, identify the
Q70: Which type of intermolecular interaction exists for
Q71: For molecules or atoms with the same
Q72: Ion-dipole forces always require_<br>A)an ion and a
Q74: The boiling point of HBr is higher
Q75: Which statement about vapor pressure of a
Q76: Define the term vapor pressure.
Q77: Which of the following statements correctly characterizes
Q78: Which of the following substances has a