Multiple Choice
Consider the phase diagram for a substance shown here. The line between the solid and liquid phases has a positive slope because the solid phase is ________ than the liquid phase. 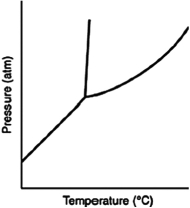
A) more dense
B) less dense
C) more massive
D) less massive
E) hotter
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q29: For molecules, atoms, or ions with the
Q30: The relative energies (strengths) of the intermolecular
Q31: Which of the following compounds will have
Q32: If the same liquid is present in
Q33: Which of the following molecules has the
Q35: The relative energies (strengths) of the intermolecular
Q36: Which of the following polar compounds is
Q37: Why does HI boil at a higher
Q38: Which of the following compounds is capable
Q39: CH<sub>2</sub>F<sub>2</sub> has a dipole moment of 1.93