Multiple Choice
The phase diagram for carbon dioxide is shown below. What is the phase that exists at room temperature (22oC) and 100 atm pressure? 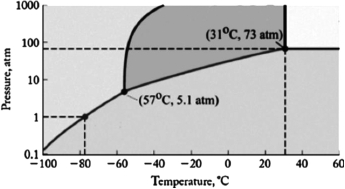
A) gas
B) liquid
C) solid I
D) supercritical fluid
E) solid II
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q90: Boiling points increase in the order HCl
Q91: When two liquids mix completely in all
Q92: When sodium chloride dissolves in water, how
Q93: Which is the dominant interaction that explains
Q94: The vapor pressure of a liquid increases
Q96: Sublimation occurs in going from region _
Q97: Which molecule has the largest dipole?<br>A)fluorine gas
Q98: CH<sub>2</sub>F<sub>2</sub> has a dipole moment of 1.93
Q99: Which is the dominant interaction between water
Q100: Which alkane compound has the lowest boiling