Multiple Choice
The relative energies (strengths) of the intermolecular forces present in each of four different pure substances are shown in the figure below. Which substance has the lowest melting point? 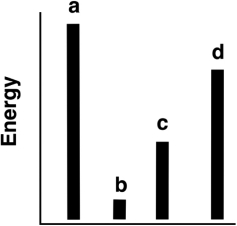
A) a
B) b
C) c
D) d
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q74: The boiling point of HBr is higher
Q75: Which statement about vapor pressure of a
Q76: Define the term vapor pressure.
Q77: Which of the following statements correctly characterizes
Q78: Which of the following substances has a
Q80: Identify the dominant intermolecular interaction(s) that must
Q81: A substance that is _ will be
Q82: A phase diagram shows the states of
Q83: Indicate which of the following molecules exhibits
Q84: For each of the following pairs of