Multiple Choice
Given the van der Waals a constant values for the following gases, which gas has the greatest intermolecular forces?
A) N2 a  1.35 L2 · atm/mol
1.35 L2 · atm/mol 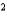
B) O2 a  1.36 L2 · atm/mol
1.36 L2 · atm/mol 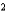
C) NH3 a  4.25 L2 · atm/mol
4.25 L2 · atm/mol 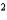
D) CH4 a  2.27 L2 · atm/mol
2.27 L2 · atm/mol 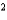
E) SO2 a  6.71 L2 · atm/mol
6.71 L2 · atm/mol 
Correct Answer:

Verified
Correct Answer:
Verified
Q80: Identify the dominant intermolecular interaction(s) that must
Q81: A substance that is _ will be
Q82: A phase diagram shows the states of
Q83: Indicate which of the following molecules exhibits
Q84: For each of the following pairs of
Q86: Molecular nitrogen (N<sub>2</sub>) interacts with water and
Q87: Of the two compounds shown below, one
Q88: Hydrophilic substances _<br>A)are immiscible in water.<br>B)are insoluble
Q89: Which is the dominant interaction between chloroform
Q90: Boiling points increase in the order HCl