Multiple Choice
Select which of the following repulsive interactions between electron pairs would be greatest.
A) 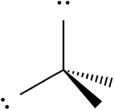
B) 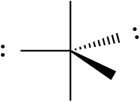
C) 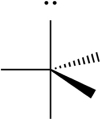
D) 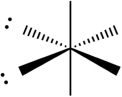
E) All involve equal repulsive forces.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q64: Identify the hybridization of the three carbon
Q65: Which type of molecular orbital has only
Q66: Predict the bond angle indicated in SF<sub>4</sub>.
Q67: When Si is doped with P, it
Q68: In the following reaction, what is the
Q70: Which of the following compounds has the
Q71: Both cyclohexane (C<sub>6</sub>H<sub>12</sub>) and benzene (C<sub>6</sub>H<sub>6</sub>) have
Q72: Identify the hybridization of atomic orbitals for
Q73: What are the hybridizations of the carbon
Q74: Which of the following materials could be