Multiple Choice
Select which of the following repulsive interactions between electron pairs would be smallest.
A) 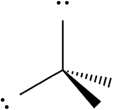
B) 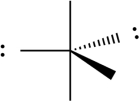
C) 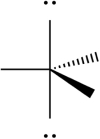
D) 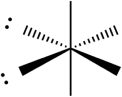
E) All involve equal repulsive forces.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q38: In the following reaction, what is the
Q39: Both ethane (C<sub>2</sub>H<sub>6</sub>) and ethylene (C<sub>2</sub>H<sub>4</sub>) have
Q40: In 1995 a Japanese cult attacked the
Q41: According to band theory, when the lower
Q42: The bonding in solid-state metals can be
Q44: Arrange the molecules below from lowest to
Q45: Use MO theory to predict the bond
Q46: Which of these molecules is chiral? <img
Q47: Of the following molecules (O<sub>3</sub>, SCl<sub>2</sub>, SO<sub>2</sub>,
Q48: A _ geometry corresponds to a steric