Multiple Choice
Which of these molecules have a dipole moment? 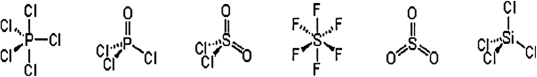
A) PCl5 and SiCl4
B) POCl3, SO2Cl2, and SO3
C) POCl3 and SO2Cl2
D) PCl5, POCl3, SOCl2, SO3, and SiCl4
E) PCl5, SF6, SO3, and SiCl4
Correct Answer:

Verified
Correct Answer:
Verified
Q145: When Ge is doped with Ga, it
Q146: What is the hybridization of the iodine
Q147: Which of these molecules do not have
Q148: The correct H-N-H bond angle in NH<sub>3</sub>
Q149: Which of the following molecules has a
Q151: Which of these molecules is chiral? <img
Q153: Which of the following statements about bonds
Q154: Identify the polar molecule.<br>A)CF<sub>4</sub><br>B)SiH<sub>4</sub><br>C)CHCl<sub>3</sub><br>D)CS<sub>2</sub><br>E)CO<sub>2</sub>
Q155: Which bond is the least polar?<br>A)H-C<br>B)H-N<br>C)H-O<br>D)H-Cl<br>E)H-F
Q180: Predict the bond order of the NO<font