Multiple Choice
Which statement concerning benzene is not correct? 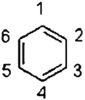
A) A second resonance structure is needed to describe benzene.
B) All the bonds originate from p atomic orbitals.
C) Benzene does not have a dipole moment.
D) The carbon atomic orbitals are sp2 hybridized.
E) Benzene has both long and short carbon-carbon bonds.
Correct Answer:

Verified
Correct Answer:
Verified
Q75: Which of these molecules is chiral? <img
Q76: Which of the following molecules is diamagnetic?<br>A)F<sub>2</sub><br>B)B<sub>2</sub><br>C)O<sub>2</sub><br>D)C<sub>2</sub><font
Q77: Identify the hybridization of the three carbon
Q78: Which element would be used to dope
Q79: GaAs and AlGaAs<sub>2</sub> are examples of _
Q81: Which electron-pair geometry corresponds to a steric
Q82: Which statement A-D about sigma (<font face="symbol"></font>)
Q83: Draw the band gap profile for a)
Q84: Which statement about a semiconductor is not
Q85: Which of the following elements are semiconductors?