Multiple Choice
The local molecular geometry and hybridization around the labeled carbon atom in the following molecule are ________ 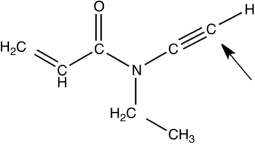
A) trigonal bipyramidal and sp.
B) trigonal planar and sp3.
C) trigonal planar and sp2.
D) tetrahedral and sp3.
E) linear and sp.
Correct Answer:

Verified
Correct Answer:
Verified
Q164: What is the molecular geometry around a
Q165: When Ge is doped with Ga, it
Q166: Draw the correct orientation of the dipole
Q167: Which element would be used to dope
Q168: Which statement about sigma (<font face="symbol"></font>) and
Q170: Which electron-pair geometry has the largest electron-electron
Q171: Arrange the interactions between pairs of electrons
Q172: Which one of the following statements regarding
Q173: For which of the following molecules is
Q174: What type of hybridization is needed to