Multiple Choice
Oxygen has two common molecular anions: peroxide (O22) and superoxide (O2) . Use the MO energy level diagram below to identify which one of the following statements is not correct. These molecular orbitals are formed from the 2s and 2p atomic orbitals. 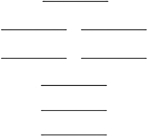
A) The bond order of the peroxide is 1.
B) The superoxide has a shorter bond than the peroxide.
C) Like O2, the peroxide is paramagnetic.
D) The superoxide has a stronger bond than the peroxide.
E) Oxygen, O2, has a stronger bond than either of these oxides.
Correct Answer:

Verified
Correct Answer:
Verified
Q23: Boron nitride is being investigated in frontier
Q24: Which statement A-D regarding semiconductors is not
Q25: What angles are present between atoms in
Q26: Using the energy-level diagram for valence orbitals
Q27: Which one of the statements A-D about
Q29: The amide structure is the fundamental linking
Q30: What is the hybridization of the bromine
Q31: For which of the following molecules is
Q32: Which of the following ions is linear?<br>A)SCN<font
Q33: Which electron-pair geometry corresponds to a steric