Multiple Choice
According to the molecular orbital energy-level diagram below, which one of the following statements is not correct about NO, NO, and NO? These molecular orbitals are formed from the 2s and 2p atomic orbitals. 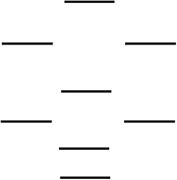
A) The bond order in NO is 2.5.
B) NO has the shortest bond.
C) Only one of these species is paramagnetic.
D) The bond order in NO is 2.0.
E) NO has the weakest bond.
Correct Answer:

Verified
Correct Answer:
Verified
Q1: For which one of the following molecules
Q2: Which of the following diagrams shows the
Q3: Which of the following compounds has a
Q4: Light-emitting diodes are semiconductors that emit light
Q6: Use the following molecular orbital diagram for
Q7: Which of the following molecules or ions
Q8: Which of the following diagrams shows the
Q9: For the molecule CH<sub>3</sub>CHCHCH<sub>3</sub>, the local molecular
Q10: Which type of molecular orbital has maximum
Q11: What hybridization is needed to describe the