Essay
Predict the indicated bond angles (less than, exactly, or greater than) in each of the following molecules. 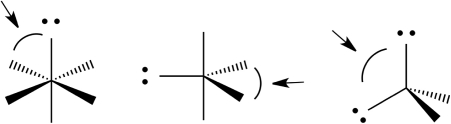
Correct Answer:

Verified
 Greater than 90° Le...
Greater than 90° Le...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q153: Which of the following statements about bonds
Q154: Identify the polar molecule.<br>A)CF<sub>4</sub><br>B)SiH<sub>4</sub><br>C)CHCl<sub>3</sub><br>D)CS<sub>2</sub><br>E)CO<sub>2</sub>
Q155: Which bond is the least polar?<br>A)H-C<br>B)H-N<br>C)H-O<br>D)H-Cl<br>E)H-F
Q156: Which bond angle is the smallest?<br>A)H-C-H in
Q157: What is the valence electron molecular orbital
Q159: What is the molecular geometry of SF<sub>4</sub>?<br>A)tetrahedral<br>B)square
Q160: Which statement regarding a <font face="symbol"></font> bond
Q161: What is the hybridization of the oxygen
Q162: Which of the following molecules or ions
Q163: The tetrahedral bond angle is _<br>A)90<font face="symbol"></font>.<br>B)180<font