Multiple Choice
How many lone-pair electrons are on the central oxygen atom in the Lewis structure for dinitrogen pentoxide (N2O5) ? A skeleton of the molecule is provided below. 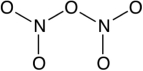
A) 0
B) 2
C) 4
D) 1
E) 3
Correct Answer:

Verified
Correct Answer:
Verified
Q138: In a chemical reaction, bonds are broken
Q139: How many valence electrons does S<sup>2</sup><sup>-</sup> have?<br>A)8<br>B)6<br>C)16<br>D)4<br>E)14
Q140: In the following molecule, identify a) the
Q141: Which of the following has the most
Q142: The measure of an atom's ability to
Q144: Nitrite (NO<sub>2</sub><font face="symbol"><sup></sup></font>) is an important nutrient
Q145: When sulfur-containing fuels are burned, sulfur trioxide
Q146: Draw the Lewis structure for the oxalate
Q147: In which bond does the Cl atom
Q148: How many shared electron pairs are there