Multiple Choice
Which group of elements is listed in order of increasing electronegativity?
A) F 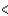 Cl
Cl 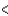 Ge
Ge 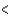 Sn
Sn
B) Rb 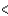 Ca
Ca 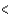 Sc
Sc 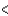 Cs
Cs
C) Zr 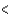 V
V 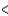 Nb
Nb 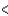 Ta
Ta
D) Sn 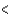 P
P 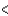 Cl
Cl  F
F
E) Cl 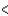 Br
Br  I
I  F
F
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q21: Which statement provides the best description of
Q22: Which of the following is a correct
Q23: Which of the following single bonds would
Q24: Indicate which of the following molecules has
Q25: In general, resonance _ electrons and _
Q27: Which of the following molecules does not
Q28: Rank the following species in order of
Q29: In addition to CO<sub>2</sub>, which of the
Q30: How many valence electrons are there in
Q31: Which of the following gases in the