Multiple Choice
Use the formal charge as a criterion to identify the Lewis structure below that is most stable.
A) 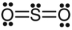
B) 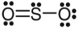
C) 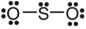
D) 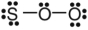
E) 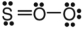
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q11: Indicate which of the following molecules has
Q12: Which of these statements correctly describes the
Q13: Which statement about the most stable Lewis
Q14: How many valence electrons are there in
Q15: To absorb infrared radiation, a molecule must
Q17: Indicate which molecule contains the largest number
Q18: Which of the following compounds would have
Q19: Indicate the element with the largest electronegativity.<br>A)K<br>B)Cs<br>C)Mg<br>D)B<br>E)Li
Q20: A covalent bond results when _<br>A)electrons are
Q21: Which statement provides the best description of