Multiple Choice
Based on consideration of formal charges, which of the following is the most stable Lewis structure for the azide ion (N3) ?
A) 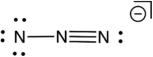
B) 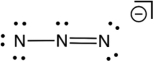
C) 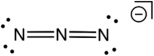
D) 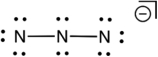
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q46: Identify which of the following bonds is
Q47: Which of the following molecules cannot absorb
Q48: What types of bonds form between the
Q49: Which of the following represents the best
Q50: Two reasonable Lewis structures for sulfurous acid
Q52: In going down a column in the
Q53: Which type of bonding involves the sharing
Q54: Which of the following ions does not
Q55: Which diatomic molecule has the least number
Q56: What is the formal charge on the