Multiple Choice
Which of the following represents the best Lewis structure for dinitrogen oxide?
A) 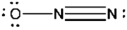
B) 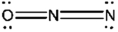
C) 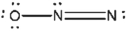
D) 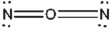
E) 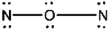
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q95: Which one of the molecules below has
Q96: Based on electronegativities, which bond of the
Q97: How many bonding electrons are assigned to
Q98: Which of the following elements has 6
Q99: Which of the following is most likely
Q101: Which of the following are listed in
Q102: What type of bonding is present in
Q103: Identify the molecule below that contains a
Q104: Which of these statements about greenhouse gases
Q105: Which one of the following molecules violates