Multiple Choice
An atom in its ground state absorbs a single photon of light and then relaxes back to the ground state by emitting an infrared photon (1,200 nm) followed by an orange photon (600 nm) . What is the wavelength of the photon that was absorbed initially? 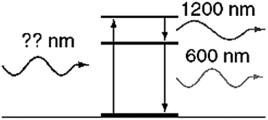
A) 600 nm
B) 1,200 nm
C) 1,800 nm
D) 900 nm
E) 400 nm
Correct Answer:

Verified
Correct Answer:
Verified
Q145: What is the energy (E, in J)
Q146: Define effective nuclear charge. Use this parameter
Q147: A light detector based on the photoelectric
Q148: The mathematical description of an electron as
Q149: Which monatomic ion most likely does not
Q151: The electron configuration of a manganese ion
Q152: How many orbitals that have the principal
Q153: What is the wavelength of a photon
Q154: Provide three potential quantum numbers for the
Q155: Which of the following occurs only in