Multiple Choice
The energy of a one-electron atom is given by 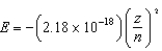 where Z is the atomic number of the element. Which of the following one-electron ions has the largest ionization energy?
where Z is the atomic number of the element. Which of the following one-electron ions has the largest ionization energy?
A) Li2
B) Be3
C) He
D) B4
E) C5
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q151: The electron configuration of a manganese ion
Q152: How many orbitals that have the principal
Q153: What is the wavelength of a photon
Q154: Provide three potential quantum numbers for the
Q155: Which of the following occurs only in
Q157: The Fraunhofer lines are evidence that _<br>A)atoms
Q158: The atomic radius of germanium (Z <img
Q159: What color will a red object appear
Q160: Which transition metal ion has no d
Q161: Which of the following statements is not