Multiple Choice
Which statement about the quantum numbers that identify an atomic orbital is not correct?
A) The magnetic quantum number, ml, identifies the orientation of the orbital in space.
B) The magnetic quantum number can have values that range from 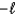 to
to 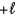 in integer steps.
in integer steps.
C) An s orbital of any given shell has only one possible ml value.
D) For p orbitals of any given shell, there are five possible ml values.
E) Statements A-D are all correct.
Correct Answer:

Verified
Correct Answer:
Verified
Q116: What is the minimum frequency of a
Q117: What is the ground-state electron configuration of
Q118: Which of the following represents a p
Q119: Which statement about the quantum numbers that
Q120: What color will a blue object appear
Q122: Which statement A-D about the wave function
Q123: A shell is defined by _<br>A)the <img
Q124: Which of the following electrons will have
Q125: Determine the wavelength of the line in
Q126: Recently, buckyballs (C<sub>60</sub>) became the largest objects