Multiple Choice
Determine the enthalpy for the following reaction, given 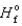 (NH3(g) ) 46.1 kJ/mol,
(NH3(g) ) 46.1 kJ/mol, 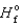 (NO(g) )
(NO(g) ) 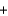 90.3 kJ/mol, and
90.3 kJ/mol, and 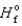 (H2O(g) ) 241.8 kJ/mol. 4NH3(g)
(H2O(g) ) 241.8 kJ/mol. 4NH3(g) 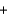 5O2(g)
5O2(g)  4NO(g)
4NO(g) 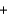 6H2O(g) Hrxn ? kJ
6H2O(g) Hrxn ? kJ
A) (1274 kJ/mol)
B) (1,996 kJ/mol)
C) (1,274 kJ/mol)
D) (905.2 kJ/mol)
E) (105.4 kJ/mol)
Correct Answer:

Verified
Correct Answer:
Verified
Q39: The diagram below shows three ion pairs:
Q40: A cooling curve for some substance is
Q41: During a(n) _ process, energy is transferred
Q42: Write the equation that corresponds to the
Q43: A heating curve for some substance is
Q45: Ethanol (CH<sub>3</sub>CH<sub>2</sub>OH) has been suggested as an
Q46: According to the first law of thermodynamics,
Q47: In a steam engine, steam in a
Q48: The enthalpy of combustion of table sugar
Q49: Benzoic acid is used to determine the