Multiple Choice
A medical saline solution is prepared by mixing 55 mg of morphine (C17H19NO3) with water to make 75.0 mL of solution. What is the millimolar concentration of this solution?
A) 1.45 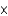
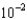 mM
mM
B) 2.57 mM
C) 3.69 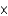
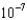 mM
mM
D) 14.5 mM
E) 2.09 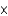
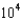 mM
mM
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q87: What is the molar concentration of sodium
Q88: In which compound does chlorine have an
Q89: The World Health Organization recommends that the
Q90: A concentrated aqueous ammonia solution has a
Q91: What is the oxidation number of P
Q93: Which one of the following statements regarding
Q94: What mass of lead(II) chloride is produced
Q95: What mass of potassium hydroxide is needed
Q96: Assuming that the density of water is
Q97: What mass of potassium iodide (molar mass